Rejecting Purdue Pharma’s Bankruptcy Plan Harms Pain Patients, Again
/By Crystal Lindell
Turns out the family behind Purdue Pharma wasn’t always acting on the up and up when it came to their money — a revelation that surprises almost no one. But a recent Supreme Court decision punishing them for that has the potential to prolong — and even cause — more suffering for millions of pain patients.
In short, last week the Supreme Court ruled 5-4 that it was wrong for the Sackler family, which owns Purdue, to essentially try to shield some of their money through bankruptcy proceedings. Under a proposed bankruptcy plan, Purdue agreed to settle a massive lawsuit over the fraudulent marketing of the opioid medication OxyContin, which they claimed was less addictive than other opioids.
Specifically, according to an NPR article about the decision, "The ruling upended a carefully-crafted settlement worth roughly $8 billion… (for) all the individuals, states and local governments that had sued over harms from the opioid epidemic.”
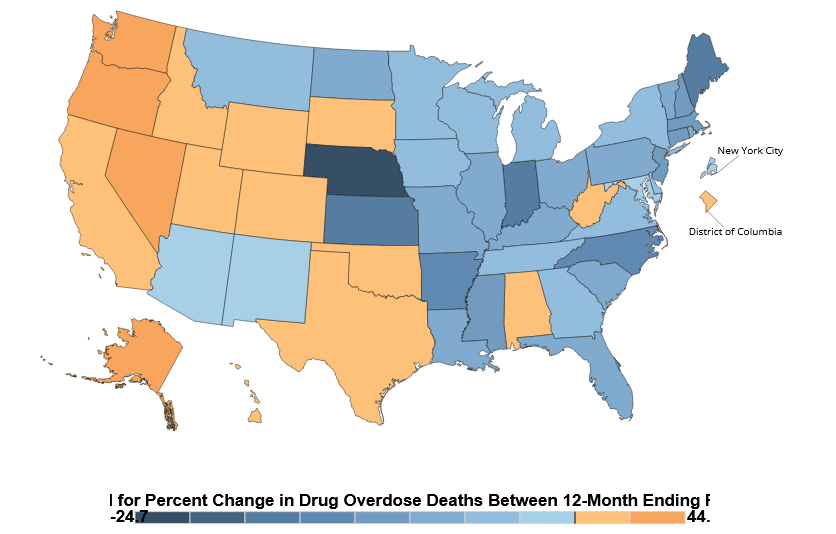
The high court’s ruling means the Sackler family is now open to more lawsuits against it, and that some of the previously decided opioid cases could now be re-opened. That’s not just bad for those slated to receive money from those lawsuits, it’s also bad for pain patients. Continuing the opioid lawsuits will only perpetuate the anti-opioid zealotry that’s infiltrated the medical community.
To be honest, on a broad level, I kind of agree with the Supreme Court. If you lose or settle a lawsuit, you should not be able to move your money around by filing for bankruptcy to shield it. The problem I have with the ruling is that it is only going to serve to prolong the failed and harmful strategy of trying to solve opioid-related problems with lawsuits.
The lawsuits are especially damaging because they perpetuate the myth that the biggest sin Purdue committed in regard to OxyContin was claiming the medication wasn’t as addictive as other opioids.
That myth is even referenced on in the Supreme Court opinion:
“Because of the addictive quality of opioids, doctors had traditionally reserved their use for cancer patients and those ‘with chronic diseases.’ But OxyContin, Purdue claimed, had a novel ‘time-release’ formula that greatly diminished the threat of addiction. On that basis, Purdue marketed OxyContin for use in ‘a much broader range’ of applications, including as a ‘first-line therapy for the treatment of arthritis.’”
However, as a pain patient myself, and also as a former OxyContin user, I am here to tell you the truth: Purdue’s biggest sin wasn’t lying about how addictive OxyContin was. No, Purdue’s biggest sin was that they claimed that OxyContin time-released pills lasted 12 hours. In reality they only last about 4-6 hours.
Don’t take my word for it though. The Los Angeles Times reported the same thing in 2016.
“The drugmaker Purdue Pharma launched OxyContin two decades ago with a bold marketing claim: One dose relieves pain for 12 hours, more than twice as long as generic medications… [But] the drug wears off hours early in many people,” the Times said.
Purdue’s lie meant that thousands of patients were not prescribed enough pills to get through the day or the month, leading to two likely outcomes.
In one scenario, patients took an OxyContin when their last one wore off, and then ran out of their medication days or even weeks before their next refill date. They then faced the impossible choice of debilitating withdrawal or seeking medication on the black market.
The second scenario is that they took the medication as prescribed, only every 12 hours, and that meant they went through daily cycles of short bursts of pain relief followed by hours of pain while they wait for their next dose.
The Times also reported that Purdue was very aware of these possible problems, but wanted to maintain the lie that OxyContin lasted 12 hours to make it stand apart from less expensive opioids. Purdue told doctors to stick to the 12-hour dosing schedule and to prescribe stronger doses if patients complained.
Here’s the thing, the way to fix the real lie -- about how long the pills last -- is to give patients more opioids, not fewer. So instead of prescribing two 10mg OxyContin per day, the doctor should prescribe four to six 10mg OxyContin per day.
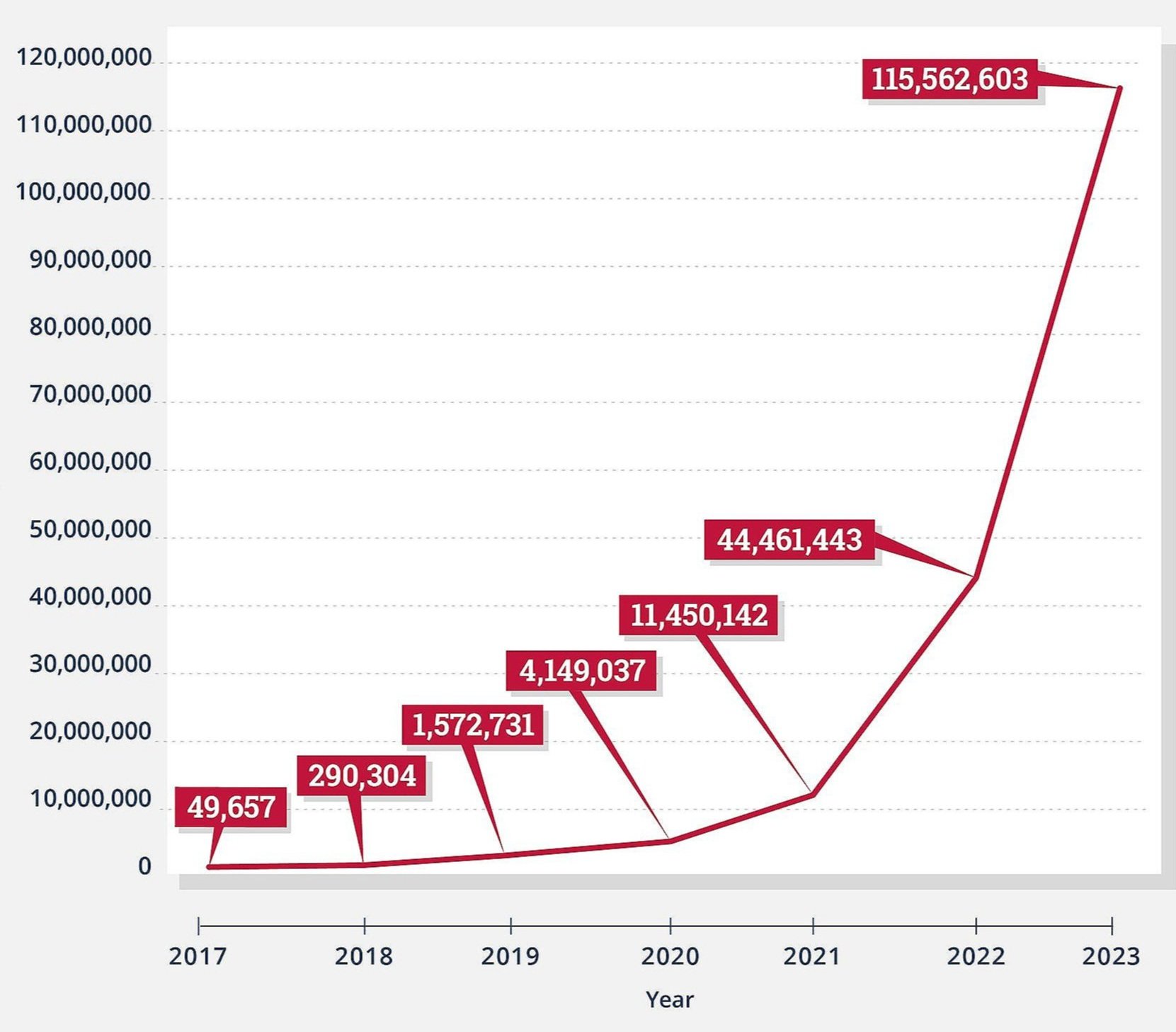
Unfortunately, that is not the lesson doctors learned from OxyContin and the opioid lawsuits. Instead, doctors decided the best solution was to minimize prescribing any opioids to any patient. As long as these lawsuits continue, medical professionals and law enforcement will be flooded with even more propaganda about how the best way to save lives is to limit opioids.
Maybe one day, we will finally realize just how damaging it has been to make people suffer needlessly by limiting opioid prescriptions. But I fear that as long as the opioid lawsuits continue, that day will be pushed further and further out into the future.
Patients know firsthand that these lawsuits have made many doctors and pharmacists scared of prescribing opioids, even for post-op pain. But opioids are still the only effective treatment for many painful conditions. This leaves patients to languish in suffering or resort to the black market for needed relief.
We could do better than that though. We could actually help people.