By Judith Graham, Kaiser Health News
Larry McMahon, who turns 80 this month, is weighing whether to undergo a major surgery. Over the past five years, his back pain has intensified. Physical therapy, muscle relaxants, and injections aren’t offering relief.
“It’s a pain that leaves me hardly able to do anything,” he said.
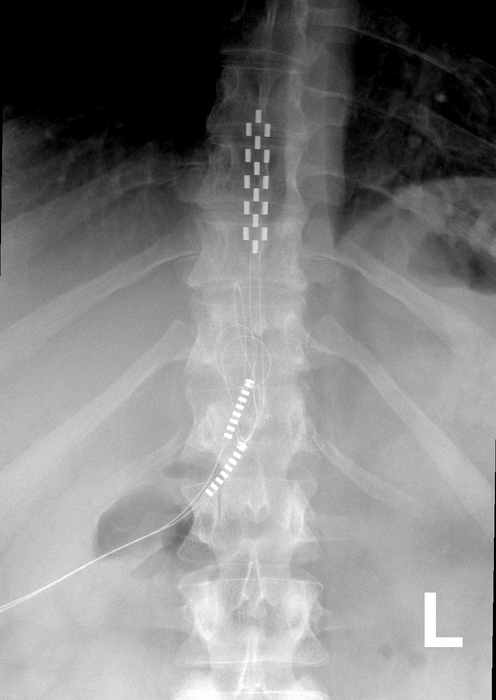
Should McMahon, a retired Virginia state trooper who now lives in Southport, North Carolina, try spinal fusion surgery, a procedure that can take up to six hours? (Eight years ago, he had a lumbar laminectomy, another arduous back surgery.)
“Will I recover in six months — or in a couple of years? Is it safe for a man of my age with various health issues to be put to sleep for a long period of time?” McMahon asked, relaying some of his concerns to me in a phone conversation.
Older adults contemplating major surgery often aren’t sure whether to proceed. In many cases, surgery can be lifesaving or improve a senior’s quality of life. But advanced age puts people at greater risk of unwanted outcomes, including difficulty with daily activities, extended hospitalizations, problems moving around, and the loss of independence.
I wrote in November about a new study that shed light on some risks seniors face when having invasive procedures. But readers wanted to know more. How does one determine if potential benefits from major surgery are worth the risks? And what questions should older adults ask as they try to figure this out? I asked several experts for their recommendations. Here’s some of what they suggested.
1) What’s the goal of this surgery?
Ask your surgeon, “How is this surgery going to make things better for me?” said Margaret “Gretchen” Schwarze, an associate professor of surgery at the University of Wisconsin School of Medicine and Public Health.
Will it extend your life by removing a fast-growing tumor? Will your quality of life improve by making it easier to walk? Will it prevent you from becoming disabled, akin to a hip replacement?
If your surgeon says, “We need to remove this growth or clear this blockage,” ask what impact that will have on your daily life. Just because an abnormality such as a hernia has been found doesn’t mean it has to be addressed, especially if you don’t have bothersome symptoms and the procedure comes with complications, said Drs. Robert Becher and Thomas Gill of Yale University, authors of that recent paper on major surgery in older adults.
2) If things go well, what can I expect?
Schwarze, a vascular surgeon, often cares for patients with abdominal aortic aneurysms, an enlargement in a major blood vessel that can be life-threatening if it bursts.
Here’s how she describes a “best case” surgical scenario for that condition: “Surgery will be about four to five hours. When it’s over, you’ll be in the ICU with a breathing tube overnight for a day or two. Then, you’ll be in the hospital for another week or so. Afterwards, you’ll probably have to go to rehab to get your strength back, but I think you can get back home in three to four weeks, and it’ll probably take you two to three months to feel like you did before surgery.”
Among other things people might ask their surgeon, according to a patient brochure Schwarze’s team has created: What will my daily life look like right after surgery? Three months later? One year later? Will I need help, and for how long? Will tubes or drains be inserted?
3) If things don’t go well, what can I expect?
A worst case scenario might look like this, according to Schwarze: “You have surgery, and you go to the ICU, and you have serious complications. You have a heart attack. Three weeks after surgery, you’re still in the ICU with a breathing tube, and you’ve lost most of your strength, and there’s no chance of ever getting home again. Or, the surgery didn’t work, and still you’ve gone through all this.”
“People often think I’ll just die on the operating table if things go wrong,” said Dr. Emily Finlayson, director of the UCSF Center for Surgery in Older Adults in San Francisco. “But we’re very good at rescuing people, and we can keep you alive for a long time. The reality is, there can be a lot of pain and suffering and interventions like feeding tubes and ventilators if things don’t go the way we hope.”
4) Given my health, age and functional status, what’s the most likely outcome?
Once your surgeon has walked you through various scenarios, ask, “Do I really need to have this surgery, in your opinion?” and “What outcomes do you think are most likely for me?” Finlayson advised.
Research suggests that older adults who are frail, have cognitive impairment, or other serious conditions such as heart disease have worse experiences with major surgery. Also, seniors in their 80s and 90s are at higher risk of things going wrong.
“It’s important to have family or friends in the room for these conversations with high-risk patients,” Finlayson said. Many seniors have some level of cognitive difficulties and may need assistance working through complex decisions.
5) What are the alternatives?
Make sure your physician tells you what the nonsurgical options are, Finlayson said. Older men with prostate cancer, for instance, might want to consider “watchful waiting,” ongoing monitoring of their symptoms, rather than risk invasive surgery. Women in their 80s who develop a small breast cancer may opt to leave it alone if removing it poses a risk, given other health factors.
Because of Larry McMahon’s age and underlying medical issues (a 2021 knee replacement that hasn’t healed, arthritis, high blood pressure), his neurosurgeon suggested he explore other interventions, including more injections and physical therapy, before surgery. “He told me, ‘I make my money from surgery, but that’s a last resort,” McMahon said.
6) What can I do to prepare myself?
“Preparing for surgery is really vital for older adults: If patients do a few things that doctors recommend — stop smoking, lose weight, walk more, eat better — they can decrease the likelihood of complications and the number of days spent in the hospital,” said Dr. Sandhya Lagoo-Deenadayalan, a leader in Duke University Medical Center’s Perioperative Optimization of Senior Health program.
When older patients are recommended to POSH, they receive a comprehensive evaluation of their medications, nutritional status, mobility, preexisting conditions, ability to perform daily activities, and support at home. They leave with a “to-do” list of recommended actions, usually starting several weeks before surgery.
If your hospital doesn’t have a program of this kind, ask your physician, “How can I get my body and mind ready” before having surgery, Finlayson said. Also ask: “How can I prepare my home in advance to anticipate what I’ll need during recovery?”
7) What will recovery look like?
There are three levels to consider: What will recovery in the hospital entail? Will you be transferred to a facility for rehabilitation? And what will recovery be like at home?
Ask how long you’re likely to stay in the hospital. Will you have pain, or aftereffects from the anesthesia? Preserving cognition is a concern, and you might want to ask your anesthesiologist what you can do to maintain cognitive functioning following surgery. If you go to a rehab center, you’ll want to know what kind of therapy you’ll need and whether you can expect to return to your baseline level of functioning.
During the covid-19 pandemic, “a lot of older adults have opted to go home instead of to rehab, and it’s really important to make sure they have appropriate support,” said Dr. Rachelle Bernacki, director of care transformation and postoperative services at the Center for Geriatric Surgery at Brigham and Women’s Hospital in Boston.
For some older adults, a loss of independence after surgery may be permanent. Be sure to inquire what your options are should that occur.
Kaiser Health News is a national newsroom that produces in-depth journalism about health issues.