Study Finds Spinal Cord Stimulation Has No Benefit for Back Pain
/By Pat Anson, PNN Editor
A scathing new Cochrane review is raising more questions about the safety, efficacy and long-term benefits of spinal cord stimulators, medical devices that are increasingly used to treat chronic back pain.
Cochrane reviews are considered the gold standard in medical research because they use robust methodology to gather good quality evidence and reduce the impact of biased, poor-quality studies.
The review by Australian researchers concluded that spinal cord stimulation (SCS) works no better than a placebo for treating chronic low back pain, and provides little to no benefit in improving quality of life.
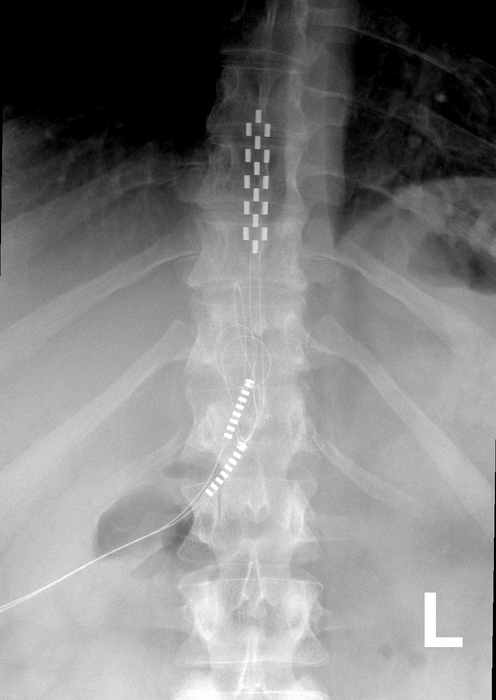
The devices are surgically placed near the spine and connected to batteries implanted under the skin, which send electrical impulses into the spine to mask pain.
“Spinal cord stimulation is invasive and has a great financial cost to people who choose surgery as a last resort to alleviate their pain. Our review found that the long-term benefits and harms are essentially unknown,” said lead author Adrian Traeger, PhD, a Research Fellow at the Institute for Musculoskeletal Health at the University of Sydney. “Our review of the clinical data suggests no sustained benefits to the surgery outweigh the costs and risks.”
Treager and his colleagues analyzed the results of 13 clinical trials of SCS devices, looking at data from almost 700 patients with low back pain. They found little to no clinical data on the long-term effectiveness of SCS because most of the studies lasted less than a month, were poorly blinded, or had selective reporting bias.
The researchers also found that side effects from SCS surgery were poorly documented, preventing them from assessing the level of risk involved. Serious adverse events include nerve damage, infection, and the devices’ electrical leads moving, all of which may lead to more surgeries.
“Data in this review do not support the use of SCS to manage low back pain outside a clinical trial. Current evidence suggests SCS probably does not have sustained clinical benefits that would outweigh the costs and risks of this surgical intervention,” they concluded.
Findings from the Cochrane review have been submitted to Australia’s Department of Health and Aged Care, which is reviewing the effectiveness of spinal cord stimulators. The devices' long-term safety and performance are also being re-assessed by Australia's Therapeutic Goods Administration (TGA).
“Our review found that the clinical benefit of adding spinal cord stimulation to treat low back pain remains unknown. When coupled with the reality that these devices are very expensive and often break down there is clearly a problem here that should be of concern to regulators,” said Chris Maher, PhD, Co-Director of Sydney Musculoskeletal Health.
Increasing Use of Stimulators
About 50,000 spinal cord stimulators are implanted annually in the U.S. and their use is growing. The devices are no longer limited to patients with back, neck and leg pain. In 2021, the FDA expanded the use of SCS to treat chronic pain from diabetic neuropathy. Stimulators are also being used on patients with Complex Regional Pain Syndrome (CRPS).
A 2018 study by investigative journalists found that SCSs have some of the worst safety records of medical devices tracked by the FDA. A 2020 FDA review of adverse events involving stimulators found that nearly a third were reports of unsatisfactory pain relief. A more recent study found that patients with the devices did not reduce their use of opioids, and continued getting procedures such as epidurals, corticosteroid injections and radiofrequency ablation.
Although evidence is growing that questions the safety and effectiveness of SCS, medical device companies continue to roll out new stimulators with more advanced technology. This week Nevro said it would release the first SCS system in the U.S. that uses artificial intelligence to optimize pain relief for each patient. The HFX iQ SCS system is designed for patients with diabetic neuropathy or chronic back and leg pain.
"This is an exciting time in spinal cord stimulation -- better waveforms, more conditions we can treat, and a massive treasure trove of patient data," said Usman Latif, MD, an interventional pain specialist and consultant to Nevro.
“What if we could take all the programming experience and clinical outcomes of tens of thousands of patients across the country, including what programs worked and what didn't, and bring the power of all that knowledge into the palm of our patient's hand -- with them 24/7, monitoring them, and offering them the best program for their exact situation with a tap on the screen. HFX iQ is the future of medicine, where expanded data holds the promise of new capabilities and improved care."
In addition to the U.S. release of HFX iQ, Nevro has asked for approval from regulators in Europe and Australia.