Miss Understood: How Arthritis Has Changed Me
/By: Arlene Grau, Columnist
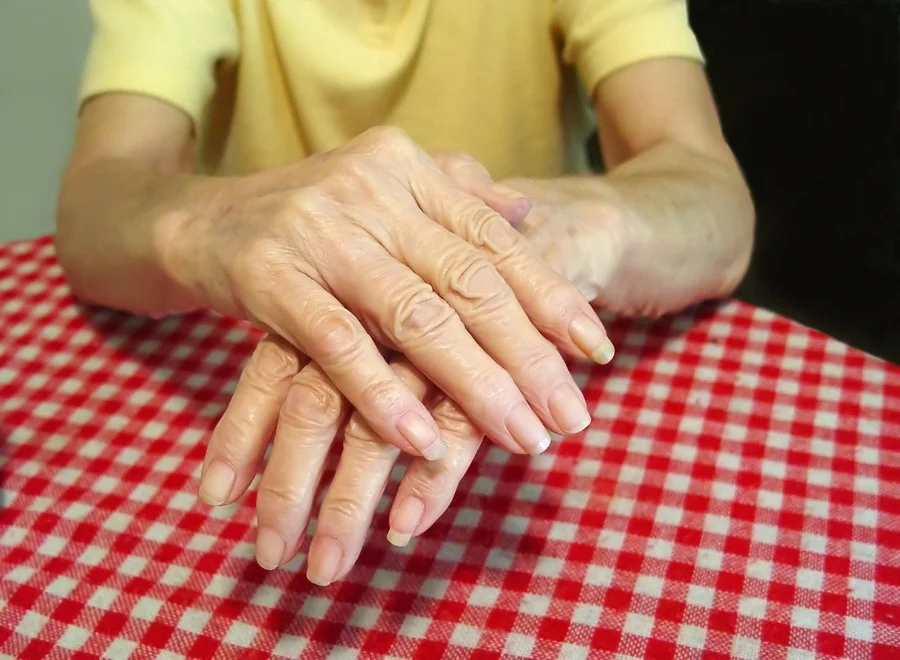
I've been noticing several changes in myself since turning 30 this past August, most of which are physical and have more to do with my lupus and rheumatoid arthritis (RA). I've never been the type of person who cares about her looks or what people think about me. However, when I began noticing large nodules forming on my fingers and persistent swelling around my wrists and knuckles I became more self-conscious.
It became especially embarrassing one day when I went to share how I had noticed certain nodules getting bigger and a friend said, "Wow that looks gross." I guess in a way I expected her to be more sympathetic about my situation, but some people may never understand.
I have some fingers that I can hardly bend and others that remain stiff for hours. Most of my fingers have become swollen and tender to the touch. I'd say my hands have suffered the most due to my RA and it makes life that much more difficult.
Just a few weeks ago I woke up unable to walk, so I ended up in the hospital. After having x-rays and an MRI, they ended up finding a labral tear and severe arthritis damage in my right hip, hence the reason why I couldn't walk.
I saw an orthopedic surgeon who said I can either have surgery now to repair it or get a cortisone injection to see if it helps temporarily, but based on the amount of damage my hip has I'm going to need a hip replacement in a few years. That news hit me like a ton of bricks.
ARLENE GRAU
I'm only thirty years old and I already have to mentally prepare myself for a future hip replacement? Not because I fell or because I broke it, but because my arthritis is so advanced that it ate away at my hip. It's a lot to take it. I feel like every time I've gotten tests done, whether its blood work or an MRI, they always find something that I don't want to hear about.
All of this and people still tell me that I don't look sick, they question my illness, or the severity of it. They question why I no longer work or what I do all day. They assume I must be having a wonderful time while my kids are at school. All assumptions because they either enjoy gossiping or they don't want to bother sitting down and getting the facts from me.
At a glance I may look like any other person. But up close you can see that I'm not your average mom or housewife.
My diseases have caused so much to my body. I have so many battle wounds and stories. Some untold, some I've cried about, and some I'm proud I've overcome.
My diseases have changed me. I'm not the same person I was when I was first diagnosed and I don't just mean that in the physical sense. In some ways I'm stronger because I've overcome so much and I'm going to continue fighting. But I also feel like I've aged and I'm tired of all the changes it's brought upon me.
They say change is good, but I don't think they were referring to the type of changes caused by autoimmune diseases.
Arlene Grau lives in southern California with her family. Arlene suffers from rheumatoid arthritis, fibromyalgia, lupus, migraine, vasculitis, and Sjogren’s disease.
The information in this column should not be considered as professional medical advice, diagnosis or treatment. It is for informational purposes only and represent the author’s opinions alone. It does not inherently express or reflect the views, opinions and/or positions of Pain News Network.