DEA Plans More Cuts in Opioid Supply, While Raising ADHD Stimulant Production
/By Pat Anson
The U.S. Drug Enforcement Administration is planning more cuts in the supply of prescription opioids in 2025, while raising production of amphetamine and other stimulants used to treat attention-deficit/hyperactivity disorder (ADHD).
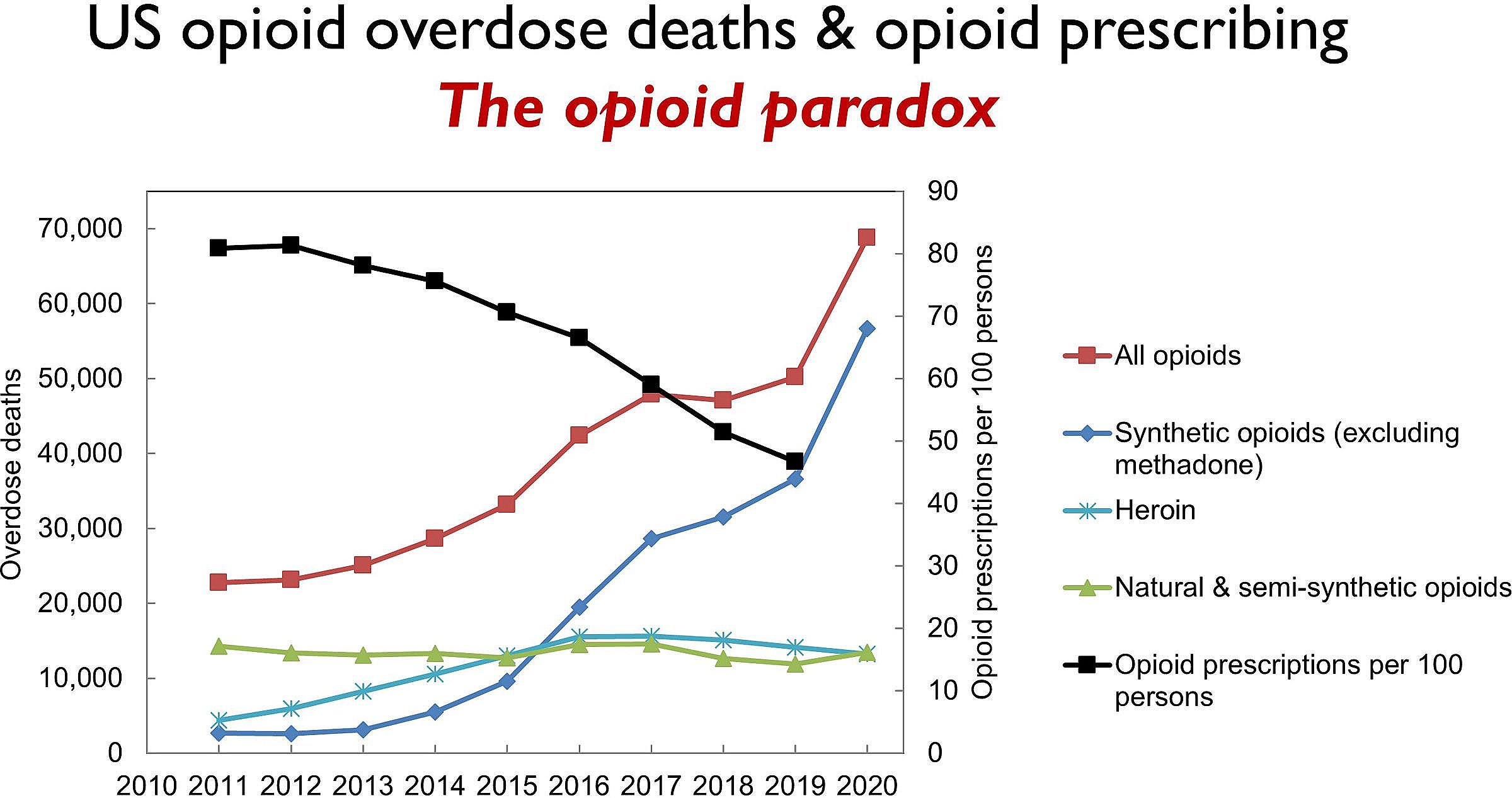
If the DEA’s plans are finalized after a public comment period, it would be the ninth consecutive year the opioid supply has been reduced.
Under the Controlled Substances Act, the DEA has broad legal authority to set annual aggregate production quotas (APQs) for drug makers – in effect telling them how much Schedule I and II chemicals and medications they can produce. DEA sets the quotas after consulting with the Food and Drug Administration (FDA) and other federal agencies, as well as individual states about the projected need for controlled substances.
“FDA predicts that levels of medical need for schedule II opioids in the United States in calendar year 2025 will decline on average 6.6 percent from calendar year 2024 levels. These declines are expected to occur across a variety of schedule II opioids. These declines are expected to occur across a variety of schedule II opioids,” the DEA said in a notice recently published in the Federal Register.
There will not be 6.6% cuts across the board for every opioid. Unlike previous years, the agency is proposing only slight reductions in the supply of fentanyl, oxycodone, hydrocodone and hydromorphone, while keeping quotas unchanged for morphine and codeine.
Most of the cuts are very minor – less than a tenth of one percent -- when the proposed APQ’s for 2025 are compared to the ones adopted in 2024.
DEA Opioid Production Quotas for 2025
Fentanyl: 0.0025% decrease
Oxycodone: 0.137% decrease
Hydrocodone: 0.081% decrease
Hydromorphone: 0.015% decrease
Morphine: Unchanged
Codeine: Unchanged
The DEA notice does not address the discrepancy between the FDA’s estimate of a 6.6% decline in medical need for opioids with the quotas it is proposing. The agency says it considered a “potential increase in demand for certain opioids” due to more elective surgeries being performed in 2025. Many of those surgeries were postponed during the COVID-19 pandemic.
The FDA predicted a 3.5% increase in domestic medical use of Schedule II stimulants in 2025. Demand for stimulants to treat ADHD has grown in recent years, but the drugs are also increasingly used to treat brain fog and fatigue caused by Long Covid. The FDA told DEA it was concerned about ongoing shortages of amphetamine, lisdexamfetamine, and methylphenidate, which are used to make stimulants such as Adderall.
The DEA wants to raise production quotas for amphetamine (+5.9%) and lisdexamfetamine (+23.5%), while leaving the 2025 quota for methylphenidate the same as it was in 2024. The agency said inventories of amphetamine and methylphenidate-based products had increased, while shortages of lisdexamfetamin continue.
“DEA believes that manufacturers will be able to meet the increase in domestic medical need for these three schedule II stimulants with the APQs proposed in this notice,” the agency said.
The Myth of Opioid Diversion
The DEA has been cutting opioid production quotas for nearly a decade, reducing the supply of oxycodone by over 68% and hydrocodone by nearly 73% since 2015. Many of those cuts are due to pressure from Congress, as well as a common belief that prescription opioids are often diverted or sold to people they are not intended for.
That belief is largely a myth.
As required by Congress, DEA estimated the diversion rate of schedule II opioids in 2025, and once again came to the conclusion that diversion is rare – less than half of one percent for oxycodone and hydrocodone. Much of the diversion is a result of theft and losses in the supply chain, before opioids even reach patients.
Estimated 2025 Diversion Rates
Oxycodone: 0.493%
Hydrocodone: 0.379%
Fentanyl: 0.013%
Hydromorphone: 0.06%
The DEA’s diversion rates are partially based on “red flag” data from prescription drug monitoring programs (PDMP). Potential red flags include patients who see three or more prescribers in a 90-day period; patients who receive a daily opioid dose in excess of 240 morphine milligram equivalents (MME); and patients who pay in cash for a controlled substance.
DEA requested PDMP data from all 50 states, but only 29 states responded to the request by sharing summaries of their red flag data.
“While PDMP data is useful in estimating diversion, it is not conclusive. Further investigation would be required before concluding that any of the subject prescriptions were actually diverted. DEA continues to evaluate its methodologies in estimating diversion in an effort to set quotas more efficiently. State participation is crucial to accurate data analysis, and DEA anticipates working closely with states, as well as other federal and state entities, in future quota determinations,” the agency said.
Public Comments
The DEA quietly published its notice about 2025 production quotas in the Federal Register on September 25, with no fanfare or press release.
Usually the agency receives thousands of comments from the public about its production quotas, but so far there have been only a handful of comments posted. Many are from patients still bitter about the decade of cuts the DEA has imposed on the opioid supply, which have contributed to record shortages of prescription drugs.
“The actions that are being taken by Congress, by the DEA, state legislatures, and state medical boards, have caused THOUSANDS of these patients to lose access to their medications, have resulted in the improper prosecution of pain doctors, resulting in a severe nationwide shortage of pain specialists, and nationwide shortages of medications,” wrote David Smith.
“The CDC, FDA, and DEA have severely underestimated the needs of chronic pain patients and misjudged the consequences of these cuts. While opioid misuse is a serious issue, penalizing legitimate pain patients is not the solution,” said Gina Harrison.
“Please I'm begging you not to cut opioids meds this year,” said Melissa Guthrie. “I'm on palliative care and there were a couple months last year I didn't get my meds due to shortages. You know it's not the pain meds causing harm. It’s the illicit fentanyl and street drugs killing people.”
You still have time to make your feelings known, as long as you do it by October 25, when the public comment period ends. To make a comment, click here.