What Is CDC Trying to Hide?
/By Pat Anson, PNN Editor
The Centers for Disease Control and Prevention made little attempt at openness and transparency when it released a draft version of its controversial opioid guideline in September 2015.
No public hearings were held. Only a select audience was invited to a secretive online webinar in which CDC officials hurriedly outlined the guideline and then refused to answer any questions about it. The guideline wasn’t posted on the CDC website and no copies were made available.
Even more puzzling is that the CDC refused to disclose who wrote the guideline or served on advisory panels such as the so-called “Core Expert Group” that played a key role in drafting the recommendations. Their names leaked out anyway.
What was the agency trying to hide?
Those issues were important five years ago, just as they are today. While the opioid guideline was only intended as a recommendation for primary care physicians treating chronic pain, it has effectively become the law of the land for all doctors in every specialty – and adopted as policy by states, insurers, pharmacy chains and law enforcement agencies.
As a result, in the name of preventing addiction, millions of pain patients have been cut off from opioids and gone without adequate pain treatment, with an untold number of suffering souls committing suicide.
Only when threatened with a lawsuit and a congressional investigation of the guideline process did the CDC back down, delaying the release of the guideline for a few months. Hearings were held, public comments were accepted, and CDC revealed the names of its experts and outside advisors, including some who had strong biases against opioids.
Five were board members of Physicians for Responsible Opioid Prescribing (PROP), a small but influential advocacy group founded by Dr. Andrew Kolodny, a psychiatrist who was then-medical director of Phoenix House, an addiction treatment chain. PROP President Jane Ballantyne, MD, and Vice-President Gary Franklin, MD, were members of the Core Expert Group, while board member David Tauben, MD, served on the CDC’s peer review panel. PROP member David Juurlink, MD, and Kolodny himself were part of a “Stakeholder Review Group” that provided input to the CDC.
Concerned about the apparent one-sided approach to the guideline, a bipartisan group of congressmen on the House Oversight and Government Reform Committee wrote a letter to then-CDC director Thomas Frieden, a longtime associate of Kolodny.
“We expect CDC’s guidelines drafting process to seek an appropriate balance between the risk of addiction and the need to address chronic pain,” wrote Chairman Jason Chaffetz (R-Utah). “The CDC has utilized a ‘Core Expert Group’ in the drafting and development of opioid prescribing guidelines, raising questions as to whether CDC is complying with FACA (Federal Advisory Committee Act).”
Chaffetz and his colleagues asked Frieden to supply documents and information about the guideline process “as soon as possible.”
‘Some Information Was Withheld’
We were curious about Frieden’s response and filed a Freedom of Information Act (FOIA) request with the CDC last year, asking for “copies of all documents, emails, memos and other communications” that the agency sent in response to Chaffetz’s letter.
The CDC’s reply, received a few weeks ago, is just as puzzling and secretive as the agency’s actions in 2015. Nearly 1,500 pages of documents provided to PNN were heavily redacted or scrubbed of all information. As a result, over 1,200 pages were completely blank.
“We located 1,449 pages of responsive records and two Excel workbooks (108 pages released in full; 103 pages disclosed in part; 1,238 pages withheld in full). After a careful review of these pages, some information was withheld from release,” Roger Andoh, who heads the CDC’s FOIA Office, wrote in a letter to PNN.
Andoh cited two FOIA exemptions to justify withholding the information. The first exemption protects material under a broad declaration of “deliberative process privilege.” Material that’s in draft form, including a reviewer’s comments and recommendations, can be withheld by the government because they are “predecisional and deliberative.”
The second FOIA exemption cited by Andoh protects information that is private because releasing it would be “a clearly unwarranted invasion of personal privacy.”
The privacy exemption was applied often to documents from a June 23, 2015 meeting of the Core Expert Group. We can see from the agenda that it was an important meeting, with clinical evidence about opioids reviewed in the morning, followed by a lengthy panel discussion in the afternoon. But we don’t know who said what because the minutes from that meeting have been deleted.
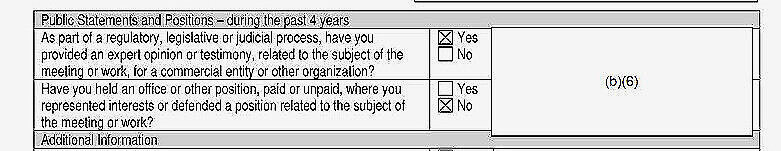
Whenever you see the notations “(b)(5)” or “(b)(6)” appear means that some information was withheld.
SOURCE: cdc foia office
The privacy exemption was also applied to the financial conflict of interest statements filed by all 17 members of the Core Expert Group (CEG). Their names and signatures were redacted, so we have no idea who they were or what conflicts they declared.
One CEG member checked a box indicating they did consulting work for “a commercial entity or other organization with an interest related to controlled substances.” Opioids are a controlled substance and so is Suboxone, an addiction treatment drug. It would be important to know who that person was, but their name was redacted, along with name of the organization they worked for.
The same individual also checked a box indicating they “provided an expert opinion or testimony.” But because the information was redacted, we don’t know if the person was paid for their testimony and, if so, who they were paid by and what the amount was.
Information was also withheld about other CEG members who were given grants, honoraria, and reimbursement for travel and lodging by organizations with an interest in controlled substances. One CEG member was actually employed by such an organization, but we don’t know who that was or who they worked for..
In short, several members of the Core Expert Group had a financial conflict of interest and disclosed it to the CDC, but the agency has decided – five years later -- that information should not be made public.
‘There Was a Cover-Up Here’
We asked three advocates in the pain community to review the documents CDC provided to PNN. All three were puzzled why so much information was withheld.
“I think what they sent is an embarrassment. There is no reasonable or rational explanation to redact any part of a suggested guideline process especially since the CDC admits the guidelines were misapplied and misinterpreted,” said Julie Killingworth, a disabled activist. “I believe the ridiculously heavy number of redactions is a clear admission of guilt. The CDC has committed at least one or multiple federal crimes and the House Oversight Committee needs to closely revisit their December 18, 2015 letter of concern to Dr. Tom Frieden.”
“There was indeed a cover-up here, grounded primarily on the escape clauses in the FOIA enabling legislation which exempts the government from revealing its internal processes or consultations to the public,” said Richard “Red” Lawhern, PhD, who heads the Alliance for the Treatment of Intractable Pain. “Unfortunately, this broad exception to full public disclosure permits agencies to hide their own biases, failures of transparency, or arbitrary decisions.
“Masking the identities of individuals who contribute to policy can also make it practically impossible to assess bias, conflict of interest, or outright misrepresentation. The extensive redacting of documents raises concern that the reviewing office has engaged in a broad cover-up by masking the identities and professional or personal affiliations of those who contributed to the CDC Guidelines."
“It could well be that there would be nothing surprising or unseemly in the redacted information. But if you don't want people to think you are trying to hide something nefarious, then the old saying that sunlight is the best disinfectant certainly would seem to apply here,” said Bob Twillman, PhD, a former executive director of the Academy of Integrative Pain Management, who was also a member of the CDC’s Stakeholder Review Group.
“It's mystifying and sad to me that CDC will not reveal who was involved in the deliberations that led to the issuance of its opioid prescribing guideline, even though they have publicly revealed much of this information elsewhere.”
Twillman points out that the identities of the Core Expert Group, as well as other advisors and contributors to the guideline, were all published in a JAMA article and by the CDC itself when the final guideline was released in 2016.
Redacting their names and conflicts of interest, as well as minutes and notes from their deliberations, is likely to fuel long-standing suspicion in the pain community that the guideline process was tainted by bias and that much of the clinical evidence was cherry-picked.
“What's worse for me is the refusal to help people understand the deliberative process that went into drafting the recommendations in the guideline,” says Twillman. “An interesting issue that is probably covered by the redacted material is the decision to reject any evidence except RCTs (randomized controlled trials) when evaluating benefits of opioids, but to accept weaker types of evidence when evaluating harms of opioids. Why did the group decide this was acceptable, and not insist on a level playing field for evidence regarding these two questions?”
The CDC recently announced plans to update and expand its opioid guideline, most likely to include the treatment of short-term, acute pain. Whether the agency will use more transparency and openness in that process remains to be seen. The updated guideline is expected in 2021.