Nerve Stimulation Effective in Treating Cluster Headache (VIDEO)
/By Pat Anson, Editor
A neuromodulation device that stimulates a nerve in the neck substantially reduced the number and frequency of attacks in patients suffering from cluster headache, according to small study published in the journal Neurology.
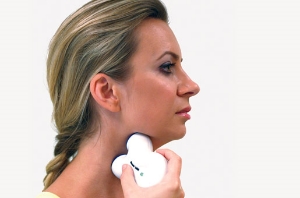
Image courtesy of electrocore
Seventy-nine percent of patients who completed the study (15 out of 19) reported an overall improvement in their condition after using gammaCore, a nerve stimulator that sends electrical signals along the vagus nerve, which runs through the neck to the brain. Eleven of the patients had chronic cluster headaches, and eight were classified as episodic.
“Cluster headache is a dreadful, extremely painful and disabling condition that can be very complex to manage. Given the unmet need for effective and safe treatments, we were excited to see the outcomes in these patients of an approach offering very considerable promise for future development.” said Peter Goadsby, PhD, who led the research at the Royal Free Hospital in London and the Beaumont Hospital in Dublin.
Cluster headache is a neurological disorder characterized by recurring, severe headaches on one side of the head, often around the eye. Attacks occur suddenly and can range from 15 minutes to three hours. Recommended treatments for cluster headaches include oxygen or triptan.
Nearly half (47%) of the acute attacks treated with gammaCore ended in an average of 11 minutes. Ten patients reduced their use of oxygen by 55% and nine patients reduced their triptan use by 48%. Preventative use of the gammaCore device resulted in a substantial reduction in the frequency of attacks, from 4.5 attacks every 24 hours to 2.6 after treatment.
The treatment, which is self-administered by the patient for two minutes, involves placing the hand-held gammaCore device on the skin of the neck over the vagus nerve. In the study, patients administered two to three rounds of neurostimulation twice per day. Acute attacks were treated with up to six doses at the onset of the attack. Patients reported no serious side events.
GammaCore, which is manufactured by New Jersey based electroCore, is not currently approved by the Food and Drug Administration and is not available in the United States.
The company is seeking FDA approval for gammaCore in the treatment and prevention of cluster headache. The device currently has regulatory approval for the acute and/or prophylactic treatment of cluster headache, migraine and medication overuse headache in the European Union, South Africa, India, New Zealand, Australia, Colombia, Brazil, Malaysia, and Canada.
“It is not certain how vagus nerve stimulation treats and prevents migraines and cluster headaches, but data suggest that it may work by sending signals into the brain that reduce the amount of a substance, called glutamate, that has been associated with headache symptoms,” the company says in a statement on its website.
ElectroCore is developing Vagus Nerve Stimulation (nVNS) therapies for the treatment of multiple conditions in neurology, psychiatry, gastroenterology and respiratory fields. The company’s initial focus is on the treatment of primary headaches (migraine and cluster headache), and the associated chronic co-morbidities of gastric motility, psychiatric, sleep, and pain disorders.
ElectroCore has raised more than $80 million from investors including Merck’s Global Healthcare Innovation fund.