Nasal Spray Approved for Migraine in Children
/By Pat Anson, Editor
The U.S. Food and Drug Administration has approved a nasal spray for the treatment of migraine in pediatric patients, the second migraine drug the agency has approved in the last month for patients 12 years of age and older.
About one in five teens suffer from migraine, but treatment options for them have been very limited compared to adults.
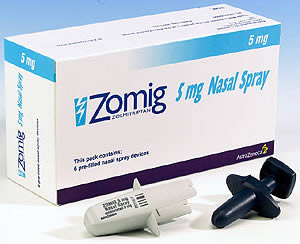
Zomig nasal spray was first approved by the FDA in 2003 for the treatment of migraine in adults. It provides pain relief in as little as 15 minutes, with most patients obtaining some relief in about two hours.
The FDA’s approval came after the agency reviewed safety and efficacy data from clinical trials demonstrating that Zomig was significantly more effective than placebo in relieving headache pain and other migraine symptoms in pediatric patients. It also had a safety profile similar to that in adults.
Zonig is the first prescription nasal spray approved for migraine in children. The most common adverse reaction to Zomig in pediatric patients during clinical trials was an unusual taste.
"Treatment options have been limited for pediatric patients and we are pleased with FDA's decision and look forward to bringing migraine relief to pediatric patients by making Zomig Nasal Spray available to this 'school age' patient population," said Fred Wilkinson, President and CEO of Impax Laboratories, which obtained the U.S. commercial rights to Zomig products from AstraZeneca in 2012. Impax has since lost exclusive rights to Zonig tablets and is focused on the nasal spray.
The recommended starting dose for Zomig in pediatric patients is 2.5 mg. The dosage can be adjusted on an individual basis, but should not exceed 5 mg in a single dose or a maximum daily dose of 10 mg in any 24 hour period.
Last month the FDA approved the migraine drug Treximet for pediatric patients 12 years of age and older.. Treximet is the first approved combination drug for migraine to contain sumatriptan and naproxen, a non-steroidal anti-inflammatory drug (NSAID). Sumatriptan is a triptan that works in the brain by reducing vascular inflammation.
Like Zomig, Treximet had already been on the market for several years to treat migraine in adults.
Migraine is thought to affect a billion people worldwide and about 36 million adults in the United States, according to the American Migraine Foundation. It affects three times as many women as men. In addition to headache pain and nausea, migraine can also cause vomiting, blurriness or visual disturbances, and sensitivity to light and sound. About half of people living with migraine are undiagnosed.
The month of June is Migraine Awareness Month.